|
|||
Post-stroke depression: Is amelioration of the quality of life possible
ZAWADZKA A.1, LESZEK J.2
1Postgratuated student in Department of Psychiatry of Medical University of Wroclaw, Poland
2Department of Psychiatry of Medical University of Wroclaw, Polande
Summary
Stroke is one of the most common cause of morbidity and mortality in Western countries and depression is a significant risk factor of morbidity in stroke patients. Appropriate diagnosis of depression following stroke is substantial benefit to person recovering from stroke by improving their medical status, enhancing quality of life (QOL), and reducing their pain and disability. There are many instruments for examining QOL, but non of them is ideal. It’s important to use our experiences with patients during diagnosis and treatment. Neurorehabilitation and adequate pharmakotherapy of PSD allow to increase QOL of stroke survivors.
Key words: Post-stroke depression (PSD), quality of life (QOL), geriatric patients.
Introduction
Stroke is the third leading cause of morbidity and mortality in the U.S.A. with an estimated annual incidence of 600.000 [National Institute of Neurological Disorders, 2001] and 60.000 in Poland (przyp. autora) [Grieb P., Stemasiak Z., 1999].
Definition of stroke includes violent prevelance of focal symptoms of brain lesion which becomes more than 24h and it has an vessel’s aetiology [Palasik W., 2003]. Up to 85% of strokes are ischaemic, i.e. a result from occlusion of an intercranial artery, there are 15% of haematic strokes, the rest of it. One of the most common cause of stroke is atreriosclerosis includes large calibre of inter -and extra cranial vessels. Stroke can occur in all age groups but three- fourths of strokes occur in people 65 years of age and over, making stroke a main cause of disability in older persons [Rockville MD, 1993]
Common behavioural and cognitive sequel of stroke include depression, psychosis, anxiety, personality changes. Depression is a major reason of morbidity in stroke patients.
One third or more of stroke victims experience significant depression and poor quality of life after stroke, according to study findings presented on opening day of the American Stroke Association’s 25th International Stroke Conference [Kerr M., 2000] or 41% in another epidemiological follow-up study [Andersen G., Vestergaard K., Riis J., et al 1994], and prevalence of depression in general population of elderly with other medical serious illness is not higher than 15% [Matt BT, MacNeill SE, Lichtenberg PA, 1999], [Fuh JL, Liu HC, Wang SJ, 1994].
Furthermore patients with PSD have higher mortality rates than patient without depression. E.g., Morris et al., following 103 consecutive stroke patient with and without depression for 10 years, found a threefold greater mortality rate in the depressed group - independent of age, sex, type of stroke lesion location and size. The mortality rate was highest for depressed patient who were socially isolated8.
Diagnosis
The diagnosis of depression following stroke is often making it difficult for aphasia, dysprosodia and anosognosia. So, that is a reason for observation of patients’ affect and behaviour provided by clinician, family and other lealthcare providers like a nursing and rehabilitation staff can be more value than information taking from a patient. E.g. patients with aphasia may be deperssed, but unable t report their feelings. Injury to the right hemisphere or frontial lobes may cause indifference, foolish and superficial affect, and lack of awareness or minimization of deficit. The amnestic patient may not recall the duration or development of depressive syndromes.
Appropriate diagnosis of depression following stroke is substantial benefit to persons recovering from stroke by improving their medical status, enhancing quality of life (QOL) and reducing their pain and disability [Bathseda MD, 2002]. Actualisation of 4th edition of the Diagnostic and Statistical Manual Version in 1994, allow for the diagnosis of depression even then patient denied feeling depressed. The criteria of both, minor and major depression, are scored based on information from patient, family, medical staff and clinician evaluation of the patient.
This rules making diagnosis of depression connected with neurological impairments, especially depression following stroke easier, more effective and what is probably most significant to be increasing in frequency [Robinson RG, 1998].
In the past there has been a tendency to ascribe potential depressiive symptoms to stroke -related side- effect [Gawronski D., Reding M.J., 2001]. But it’s important to remember that vegetative symptoms such as loss of appetite and change of sleeping patterns are non-specific, yet more often present in post-stroke depression [Paradiso S.,Onkuho T., Robinson RG, 1997].
In this connection we could distinguish two different types of approchements to diagnosis called “exclusive”; led to a 1% to 2% underdiagnosis, and “inclusive” led to an estimated 5% overdiagnosis.
Using Robinson’s RG The Clinical Neuropsychiatry of Stroke which is the most extensive, exghausting review of all issued associated with following stroke depression, we can precisely characterise criteria and for depression and their occurrence among patients with post-stroke depressive disorders [Fig. 1].
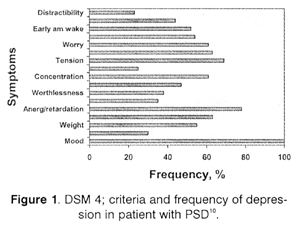
Basing on DSM-4 the diagnosis of depression requires presence of at least five symptoms for at least 2 weeks period of time. One of them must be either depressed mood or loss of interest or anhedonia.
The remaining four symptoms may include considerable weight loss or weight gain, insomnia or hypersomnia, psychomotor agitation or retardation, fatigue or loss of energy, feeling of worthlessness or excessive or inappropriate guilt, diminished ability to think or concentrate, and recurrent thoughts of death, low level of sexual intererst and activity [American Psychiatric Association, 1994].
Except for minor differences, the clinical presentation of PSD and idiopathic depressive illness are similar. Physical and mental slowness are more frequent in PSD, loss of interest and concentration, more common in idiopathic depression. Some authors suggested that patient with PSD exhibit more anxiety, worry and tear fullness, but less guilt, worthlessness and suicidal thoughts.
The effects of stroke may mimic depression or impair emotional communication.
There are also some commonly used structured interviews for estimating process of depression, like The Hamilton Depression Scale and The Present Scale Examination, and a newer scale - The Global Health Questionnaire that is most clinically useful because of its sensitivity and specificity [O’ Rourke S. et al, 1998]. There are recently also considering to identify some biologic markers of neuroendocrine changes in depression, i,e, prolactin pesponse to buspiron [Sevincok L., Erol A., 2000] or prolactin responce to d-fenfluramine [Okada K., Kobayashi S., Yamagata S., et al, 1997], but up to now they haven’t any clinical application.
Prevalence and course of PSD
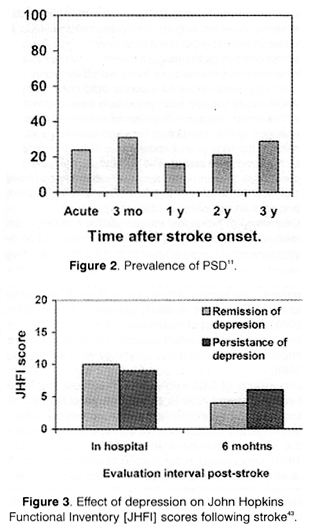
As we mentioned prevalence of PSD range from 30 to 50% depending on author. Robinson found a 27% rate of major depression and a 20% rate of minor depression in the first three months after stroke. House found that 11% had major depression at one month and 5% at one years post stroke. Majority of patients with post stroke major depression are improved at two years. Minor depressive symptoms, however, often persist beyond two years. Approximately one-third of patients who are not depressed in the immediate post-stroke period period become depressed between three months and two years following stroke. The delayed onset of PSD may be due to patients’ realization that their disability is chronic, or to functional reorganization of neurotransmitter system regulating mood cause by injury [Fig 2 and 3].
The other factors influencing the prevalence of depression following stroke are first of all age of 65 and more, and a history previous stroke, severity of stroke, presence of post stroke cognitive, and lack of involved family and friends [Hosking SG, Marsh NV, Friedman PJ, 2000], [Langhorne P. et al, 2000]11.
The frequency of depression obviously is lower in medical systems that appreciate its importance and have prophylactic counselling and supported groups [Lila M. et al, 1998].
The increase of mortality at 12 and 24 months after stroke are probably; first and foremost PSD, in accordance with House’s epidemiological examinations [House et al, 2001], stressed -related cardiac rhythm disturbance, depression- related hopelessness, inability to exercise, stop smoking and medication - compliance habits, too. Victims of stroke are particularly subjecting to falling and fall-related injures, but especially patients with PSD [Ugur C. et al, 2000].
Pathophysiology
Debatable remains correlation between the severity of stroke and the sever course of PSD. In opinion of some authors i.e. Starkstein SE, Robinson RG [Starkstein SE, Robinson RE, 1989] it isn’t associated, but according to Kellermann et al [Kellermann et al, 1999] study there is close correlation.
Injury of spesific brain areas is still controversial to increase the risk of developing PSD. The investigators conducted either magnetic resonance imaging or computer tomography scans to determine the part of brain affected, and also administrated a standardised test assessing depression and a stroke-specific quality of life scale. Evidence suggest that bilateral lesion in the anterior frontal and temporal lobes and caudate nuclei are associated with an increase risk of depression. The rate of depression is also increased in older people with diffuse subcortical microvascular disease [Thomas et al, 2001]. The risk factor of late-life depression are moreover atherosclerotic changes of large calibre vessels responsible for the leukoaryosis and subcortical atrophy [Rao R, 2000]. Some authors have posited that lesions in the left hemisphere, particularly the left anterior frontal lobe and left caudate nucleus, aremore likely to be associated with PSD. Severity of depressive symptoms in patients with right hemisphere stroke was greater that left hemisphere22. The observation of patients with left frontal strokes were more likely to have expressive aphasia with preserved awareness of their communication impairments and frustration. Those of left temporal lesion with fluent aphasia are often unaware of their communication deficit and not frustrated by them. Nevertheless analysis of relation fo lesion location to verbal and nonverbal mood measure in patients with PSD, give no support for hyppthesis that the risk of PSD is correlated with stroke location [Carson AJ et al, 2000], mainly with left frontal lesion [Gainotti et al, 1997].
Very interestig results are suggested by other studies; they state lateralized biochemical changes in responce to infraction22. Mayberg et al found that serotonin binding was higher in the right hemisphere injury than in the left anterior hemisphere after left hemisphere injury [Mayberg et al, 1994].
Robinson and Starkstein have speculated that this relative inability of the left anterior hemisphere to upregulate serotonin receptors after stroke is one reason why injury to this region is more likely to produce depression.
It is important to note that individuals with anosognosia or dysprosodia associated with right hemispheric lesion have the same frequency of PSD as those without them [Starkstein et al, 1992; 1994] and it only influence on manifestations of depression than on its frequency.
Stroke may cause a disruption in neurotransmitters, such as serotonin and noradrenaline.
Animals’ model study shown significant a decreasing in serotonine and noradrenaline after the stroke and in the hemisphere ipsilateral to the lesion came to 45% and in the intact hemisphere 29% [Robinson R et al. 1973], [Boyeson M et al, 1992]. The fact is empirically supported by a responce to treatment with noradrenaline and serotonin reuptake inhibitors.
The most of authors suppose that development of depressive neuroendocrine deregulations might caused increasing of platelet surface glycoprotein expression and because of that associated with cerebrovascular disease [Cassidy et al, 2003].
Post-stroke depression - a source of disability, suffering and decreasing QOL
As we know, the clinical assessment of patients after stroke is usually focused on the detection and determination of neurological deficits. However, the full picture of the patient after stroke should take into limitation in performing one’s social function [Brola W, Czernicki J, Szafraniec L, 1999]. Thus, assessing quality of life after stroke is an alternative to traditional methods of evaluation of the patient’s condition.
Post-stroke deperssion significantly affects the patients’ perception of their quality of life [QOL]. Impact of depressin on QOL may be greater when associated with advanced age, great severity of motor impairment or paralysis, lack of social support, inability to return to work, cognitive impairment, the presence of comorbid health problems etc [Kauhanen ML et al, 2000]. The correlation between age and QOL has reminded obsucre. The present study showed this correlation. Most authors have reported that QOL is either independent of gender or lower in females.
When comparing the married patients and the patients who were not married but living with their family, the married patients showed a poorer outcome. High levels of social support have previously been associated with a better outcome. Sometimes stroke may lead to changes in the interaction between spouses and the roles in the family, and spouse may react by overprotection and over caring. The willingness and ability of ceregivers to support stroke survivors appear to have significant influence on a patients’ emotional reaction, and on success of rehabilitation.
Decreased of functional independence has been correlated with a decreased QOL in the majority of studies reviewed. However it is vital to note that even individuals who are independent in activities of daily living report a decrease of QOL. These data support the concept that functional outcomes measures alone are not adequate for documenting stroke outcomes.
Post-stroke depression considerably affects the patients’ perception of their quality of life [Fruhward S, Loffer H, Eher R et al, 2001] [Jaracz K, Kozubski W, 2003]. The impact of depression on quality of life may be even greater when associated with cognitive impairment [Jonkman EJ, de Weerd AW, Vrijens NL, 1998].
Dimensions of QOL in people with aphasia that have been suggested include elements relating to physical problems, body image and mobility, communication and psychological, interpersonal, spiritual and financial issue. These issue in the study [LePointe LL, 1999] were placed in the context of wider dimensions of satisfaction and quality of life related to individuality, culture and philosophical and time elements, but it still stand a challenge in health related research to get satisfying scientifical model to definite measurement of QOL among people with aphasia.
Generally, the ideal instrument for measuring quality of life post-stroke currently does not exist and the advantages and limitations of the available measures have been discussing all the time. The ideal instrument for measuring quality of life post-stroke would be reliable instrument for use by either patients or their proxies and medical staff. It would be pesronsive to meaningful patient changes across a wide range of clinical presentations.
Generic utility health-related QOL instruments are useful in assessing stroke outcomes because they facilitate a broader description of disease and outcomes, allow comparison between diseases, and can be used in cost-benefit studies. There is some very valuable study that demonstrated the AQOL [Assessment of Quality of Life] include strong psychometric properties and appears to be valid and sensitive measure of health-related QOL after stroke.
In the examination of Sturm et al validity of the AQOL was assessed by examining associations between the AQOL and comparator instruments: the Medical Outcomes Short-Form Health Survey [SF-36]. London Handicap Scale, Barthel Index, National Institutes of Health Stroke Scale and Irritability, Depression, Anxiety Scale [Sturm et al, 2002].
All of those discussed scales are relatively simple and quick to administrate, but it’s necessary to keep your objectives, your available resources, and the characteristic of your patient in mind as you choose your instrument.
Treatment
The severity of physical disability to stroke doesn’t correlate well with PSD. Chronic functional impairment, however, may contribute in part to the development of PSD. Though randomised trials of active physical therapies have not demonstrated efficacy in the treatment of PSD. Neurorehabilitation remains an important part of treatment after stroke that also influence to increase of QOL [Harrington C, Salloway S, 2003].
The pharmacological treatment of depression following stroke has significant meaning and is associates with enhanced self-care independence, and cognitive function [Chemerinski E, Robinson RG, Kosier JT, 2001].
The general maxim of “start slow and go slow” in geriatric patients holds true in the treatment in PSD. The selection of anti-depressant medication for PSD is usually guided by a comparison of side-effect profiles between available medication. But pharmacokinetic interactions must also be considered when prescribing SSRI, that inhibit a number of hepatic P450 enzymes. So that SSRI may alter the metabolism of other commonly prescribed geriatric patients medications such as beta-blockers, TCAs, anticonvulsants, type IC antiarythmics, calcium channel blockers, benzodiazepines, antihistamines and certain antibiotics.
There are few controlled trials of the pharmacological treatment of PSD. A number of antidepressant have been studied for application in post-stroke depression.
The selective serotonin reuptake inhibitors [SSRI] have the safest side-effect profile.Nortryptiline, an older tricyclic medication, though in some studies was found more effective than i.e.: fluoxetine [Robinson RG, Schultz SK, Castillo C et al, 2000] have significant anticholinergic and cardiac side-effect profile. Also buproprion, a newer antidepressant is relatively free of anticholinergic. cardiac, gastrointestinal, and sexual side effect. This low side-effect profile and its structural similarity to the amphetamines make it an attractive option in the treatment of PSD. However, the increased frequency of seizures in population of patients with PSD treated with bubroprion may limit its use in PSD.
The choice of antidepressant for PSD should be based on presence of associated symptoms and disorders. A meta-analysis undertaken by the Cochrane Consortium of studies of depression in patient with organic conditions that included stroke concluded that treating deperssion is safe and effective. They estimated that one in 10 patients will drop out, and that the numberneeded to treat for every effective recovery id four to one [Gill D, Hatcher S, 2000].
Conclusions
Although the diagnosis of PSD is often complicated by anosognosia or dysprosodia, there are some instruments making it easier such as AQOL.
However, our personal knowledge with patients with depression following stroke is the most valuable, so it is important to be aware of new developments in our understanding of depression after stroke, its diagnosis, prevalence, pathopsychology, clinical, features and treatment. Observation of patient, provided by family and medical staff can be the most important and more value than information provided by patient.
The physician must stay especially vigilant up to 2 years after stroke.
REFERENCES
- Grzeb P, Stelmaski Z: Praktyczne aspekty neuroprotekcjii wudarze niedokrwiennym. Psychiatria Neurologia, 1999.
- Palasik W: Leczenie przeciwplytkwe, przeciwkrzepliwe oraztrombolityczne w udarach niedokrwiennych. Terapia, 2003.
- Rockville MD: Detection and diagnosis. Depression Guideline Panel. Clinical practice guideline, number 5, 1993.
- Ker M, Depression and stroke. Reuters Health, 2000.
- Andersen G, Vestergaard K, Riis J et al: Incidence of post-stroke depressionduring the first year in a large unselected stroke population determined using a valid standardized rating scale. Acta Psychiatrica Scandinavica, 1994.
- Matt BT, McNeill SE, Lichtenberg PA: Gerontophsychological problems in medical rehabilitation: dementia and depression among stroke and lower extermity fracture patients. J Gerontol Series A, Biol Sci & Med Sci, 1999.
- Fuh JL, Liu HC, Wang SJ, et al: Post-stroke depression among the Chinese,elderly in a rural community. Stroke, 1997.
- Morris PLP et al:Association of depression with ten-year post-stroke mortality. Am J Physiatry, 1993
- Batsheda MD: Depression and stroke. NIMH Depression Publications, 2002.
- Robinson RG et al: The Clinical Neuropsychiatry of Stroke. Cambridge University Press, 1998.
- Gawronski D, Reding MJ: Post-stroke depression: An Update. Current Arteriosclerosis Reports, 2001.
- Paradiso S, Ohkubo T, Robinson RG: Vegetative and psychological symptoms associated with depressed mood over the first two tears after stroke. Int J Psychiatry Med, 1997.
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 1994.
- O’Rourke S et al:Detecting Psychiatric morbidity after stroke; comparison of the GHQ and the HAD Scale. Stroke, 1998.
- Sevincok L, Erol A: The prolactin response to buspirone in post stroke depression; a preliminary report. J Affective Disord, 2000.
- Okada K, Kobayashi S, Yamagata S et al: Post-stroke apathy and regional cerebral blond flow. Stroke, 2001.
- Hoskings SG, Marsh NV, Friedmann PJ: Depression at 3 months post-stroke in the elderly; Predictors and indicators of prevalence. Aging Neuropsych Cognit, 2000.
- Langhorne P, Stott DJ, Robertson L et al: Medical compilations after stroke; a multicenter study. Stroke, 2001.
- Lila M et al: Depression after stroke; Results of the FINN-STROKE study. Stroke, 1998.
- House et al: Mortality at 12 and 24 months after stroke may be associated with depression symptoms at 1 month. Stroke, 2000.
- Ugur C et al: Charasteristic of falling in patient with stroke. J Neurol Neurosurg Pshchiatry, 2000.
- Starkstein SE, Robinson RG:Affective disorders and cerebral vascular disease. Br J Physiatry, 1999.
- Kellermann M, Feckete I, Gesztelyi R et al: Screeening for depressive symptoms in the actue of stroke. Gen Hospit Psychiatry, 1999.
- Thomas et al:A neyropsychological study of vascular factors in late-life depression. J Neurol Neurosurg Pshchiatry, 2001.
- Rao R: Cerebrovascular disease and late life depression: an age old association revisited. Int J Geriatr Psychiatry, 2000.
- Carson AJ et al: Depression after stroke and lesion location: a systematic review. Lancet, 2000.
- Gainotti et al: Relation of lesion location to verbal and non verbal mood measures in stroke patients. Stroke, 1997.
- Mayberg HS et al: Frontal lobe lobe disfilinction in secondary depression. J Neuropsych Clin Neuroscience, 1994.
- Starkstein et al: Anosognosia in patients with cerebrovascular lesion. A study of causative factors. Stroke, 1992.
- Starkstein et al: Neuropsychological and neuroradiological correlates of emotional prosody comprehension. Neurology, 1994.
- Robinson R et al: Effect of experimental cerebral infarction in rat brain catecholamines and behaviour. Nature, 1973.
- Boyeson et al: Unilateral but not bilateral locus ceruleus lesions facilitate recovery from sensorim-motor cortex injury. Brein Res Bull, 1992.
- Cassidy EM et al: Platelet surface glycoprotein expression in post-stroke depression; preliminary study. Psychiatry Res, 2003.
- Brola W, Czernicki Czerniaki, Szafraniec L: Examination of the quality of life in post-stroke depression. Przeglad Lekarski, 1999.
- Kauhanen ML et al: Domains and determinants of quality of life after stroke caused by brain infarction. Arch Phys Med Rehabilit, 2000.
- Kim P et al: Quality of life of stroke survivors.Qual Life Res,1999.
- Fruhwald S, Loffler H, Eher R et al: Relationship between depression, anxiety and qol. A study of stroke patients compared to chronic low back pain and myocardial ischaemia patient. Psychopathology, 2000.
- Jaracz K, Kozubski W: Quality of life in stroke patients. Acta Neurol Scand, 2003.
- Jonkman EJ et al: Quality of life after a first ischaemic stroke. Acta Neurol Scand, 1998.
- LePoint LL: Quality of life with aphasia. Semin Speech Lang, 1999.
- Sturm et al: Brief comprehensive quality of life assessment after stroke; the assessment of ql instruments in the northeast Melbourne. Stroke, 2002.
- Harrington C, Sallowey S; The diagnosis and treatment of PSD.Clinical Neuroscience, 2003.
- Cherminski E, Robinson RG, Kosier JT; Improved recovery in activities of daily living associated with remission of post stroke depression. Stroke, 2003.
- Robinson RG, Schulyz SK, Castillo C et al: Nortryptiline versus fluoxetine in the treatment of depression and in short-term recovery after stroke; a placebo-controlled, double-blind study. Am J Psychiatry, 2000.
